Certificate & test
For the Safety, to be a Guardian
Comfortable & Safe NEOSIS
Boram C&H manufactures the feminine hygiene goods with not only Quality Control, but also Quality Assaurance Dept.
For the Better product, to be the Perfect Goods
Safety Test Report
NEOSIS performs Safety Examination at Global Test Laboratory Institute called "FITI" for the aussured data based Reports
The First Step of Insurance.




Certificate registration No.
D286-16-00014, D286-16-00013
Sample request
NEOSIS Soft & Quick, NEOSIS Cotton Therapy
Examination
Ministry of Food and Drug Safety notification no.2015-33
Use
Quality control


FITI TEST REPORT
International Laboratory for Goods' Inspection
NEOSIS were certified as "Made 100% in Korea" by Korea Food & Drug Administration (KFDA)
Safe from Formaldehyde, Flourescent Agent, Coloring, Chemicals, Acid Washing
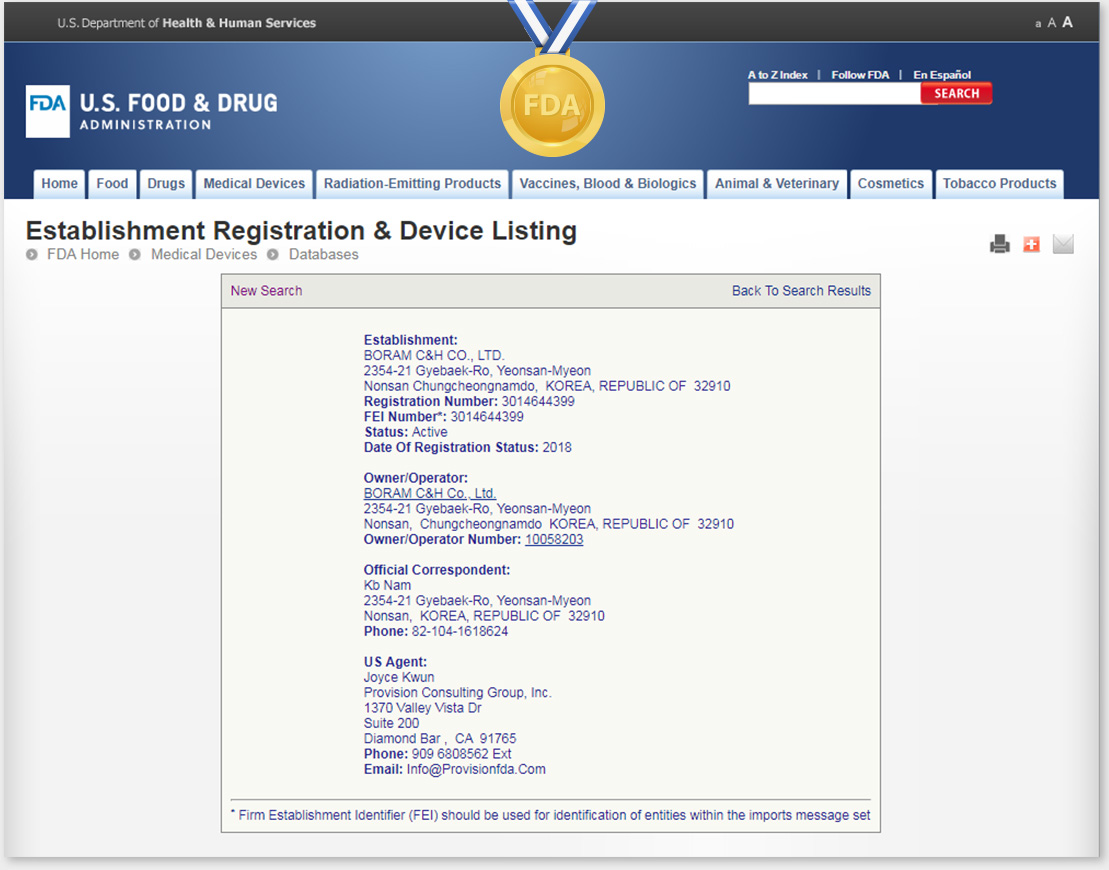
U.S. FDA Registered Manufacturer & Goods
Boram C&H is proudly registered as a official Manufacturer of Medical Device (Feminine Hygiene) by U.S. FDA.
All NEOSIS Brands are also registered as FDA Goods through U.S. FDA.


OCS: Global Certificate of Organic Cotton
NEOSIS acquired OCS certificate through strict inspection on both Systems & Management of Facility[inc. Machines] & Goods.
OCS Certificate proves that Boram C&H's Orgniac Cotton with OCS logo is from the soil with no chemical fertilizer & chemicals are used.




GB Test: Chinese National Standard Inspection on Goods
NEOSIS has passed on Chinese National Legal Sanitary Certificate called "GB Test" for Custom Inpsection in China.
This test is screening Toxicity and Micro-Organism under Chinese Quarantine Serivce for Chinese Customer's Safety.




Wating for What?
NEOSIS is here for You and Only
Various Certificates

[ ISO 9001 ]
Certiificate of Quality Management System

[ ISO 14001 ]
Certiificate of Quality Management System

[Registariton Report on Research Laboratory]
Certificate of Affiliated & Subsidiary Research Lab.

[License on Sanitary Aid Manufacturing Process]
Registration Report of Sanitary Aid Manufacturer